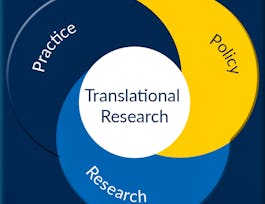
Translational science seeks to speed up the process of moving research discoveries from the laboratory into healthcare practices. Numerous scientific and organizational roadblocks can act as obstacles along the path of translation and ultimately hinder the speed of progress in medical research. The National Center for Advancing Translational Sciences (NCATS) was established by the National Institutes of Health (NIH) to transform and accelerate the translational research process, with the intended result of getting treatments to more patients faster. The field of Translational Science aims to bridge these gaps by:


Introduction to Translational Science
Taught in English
Some content may not be translated
8,722 already enrolled
(115 reviews)
What you'll learn
Students will be able to define translational science.
Students will be able to explain how translational science can accelerate research discoveries to impact healthcare practices.
Details to know

Add to your LinkedIn profile
3 quizzes
See how employees at top companies are mastering in-demand skills


Earn a career certificate
Add this credential to your LinkedIn profile, resume, or CV
Share it on social media and in your performance review

There are 3 modules in this course
The first lesson in Introduction to Translational Science provides an overview of the basic definitions and key concepts related to clinical and translational science and the CTSA program. Students will learn about the different stages of translational research and the roadblocks that impede progress across the spectrum.The second lesson in Introduction to Translational Science provides an introduction to T1 research which is research that translates findings from basic (laboratory) research to humans through developing treatments and interventions. T1 research is often conducted through observational studies, case studies, proof-of-concept studies, phase I clinical trials, and phase II clinical trials
What's included
8 videos5 readings1 quiz2 discussion prompts
The third lesson in Introduction to Translational Science provides an introduction to T2 research, which is research that translates findings from T1 research (humans) to patients. T2 research is often conducted through Phase III clinical trials, observational studies, evidence synthesis, and clinical guidelines development. The fourth lesson in Introduction to Translational Science provides an introduction to T3 research, which is research that translates findings from T2 research (patients) to actual clinical practices. T3 research is often conducted through Phase IV clinical trials, health services research, and clinical outcomes research.
What's included
9 videos6 readings1 quiz2 discussion prompts
The fifth lesson of Introduction to Translational Science provides an introduction to T4 research, which is research that translates findings from practices (T3 research) to communities, both domestic and globally. T4 research is often conducted through prevention and outcome studies, mass screening studies, and health policy studies.The sixth lesson of Introduction to Translational Science provides an overview of how interested individuals can participate in clinical and translational research; as an independent research, as a clinical research professional, and as a study volunteer. The final segment of the module demonstrates what a typical study volunteer will encounter if they are selected to participate in a clinical trial.
What's included
10 videos8 readings1 quiz2 discussion prompts
Instructor

Offered by
Recommended if you're interested in Basic Science
University of Michigan

University of Michigan

University of Michigan

Nanjing University
Why people choose Coursera for their career




Learner reviews
Showing 3 of 115
115 reviews
- 5 stars
71.30%
- 4 stars
20%
- 3 stars
2.60%
- 2 stars
4.34%
- 1 star
1.73%
New to Basic Science? Start here.

Open new doors with Coursera Plus
Unlimited access to 7,000+ world-class courses, hands-on projects, and job-ready certificate programs - all included in your subscription
Advance your career with an online degree
Earn a degree from world-class universities - 100% online
Join over 3,400 global companies that choose Coursera for Business
Upskill your employees to excel in the digital economy
Frequently asked questions
Access to lectures and assignments depends on your type of enrollment. If you take a course in audit mode, you will be able to see most course materials for free. To access graded assignments and to earn a Certificate, you will need to purchase the Certificate experience, during or after your audit. If you don't see the audit option:
The course may not offer an audit option. You can try a Free Trial instead, or apply for Financial Aid.
The course may offer 'Full Course, No Certificate' instead. This option lets you see all course materials, submit required assessments, and get a final grade. This also means that you will not be able to purchase a Certificate experience.
When you purchase a Certificate you get access to all course materials, including graded assignments. Upon completing the course, your electronic Certificate will be added to your Accomplishments page - from there, you can print your Certificate or add it to your LinkedIn profile. If you only want to read and view the course content, you can audit the course for free.
You will be eligible for a full refund until two weeks after your payment date, or (for courses that have just launched) until two weeks after the first session of the course begins, whichever is later. You cannot receive a refund once you’ve earned a Course Certificate, even if you complete the course within the two-week refund period. See our full refund policy.